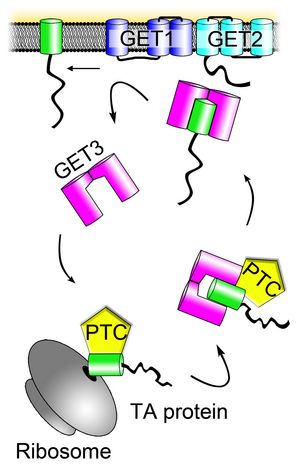
Roughly one third of the eukaryotic proteome comprises integral membrane proteins (IMPs) (Shao and Hegde. 2011). These can be further subdivided according to the number of transmembrane domains (TMD), their orientation within the membrane (Type I = cytosolic C-terminus, Type II = luminal C-terminus) and/or their chronological ribosomal processing (co- or posttranslational). The cell faces a range of biophysical challenges when translating IMPs. For example the lipid bilayer has to be penetrated to integrate the TMD of the protein in its hydrophobic core. Also, due to that hydrophobic nature of the TMD it needs to be shielded from forming potentially toxic aggregates in the hydrophilic environment of the cytosol. Most IMPs use the SEC61 translocon pore in the ER membrane where the ribosome docks after initiating translation of an IMP. The first TMD or a hydrophobic N-terminal signal sequence is recognized on the nascent amino acid chain, binds to a cytosolic receptor (SRP, signal recognition particle) which causes a translational arrest until docking at the translocon where translation resumes directly into the ER membrane or lumen (Park and Rapoport 2012). This co-translational pathway does not apply to a significant number of proteins that carry a single TMD near the C-terminus (so-called Tail-anchored (TA) proteins (Borgese et al. 2003; Kriechbaumer et al. 2009). Recently, an SRP independent pathway, Guided Entry of TA proteins (GET), has been described in yeast and humans demonstrating a cellular solution for post-translational ER insertion (Stefanovic and Hegde, 2007; Schuldiner et al. 2008 ). Here, a cytosolic pretargeting complex consisting of SGT2, GET5 and GET4 in yeast shuttles the nascent TA protein to the cytosolic ATPase GET3 (reviewed in Denic et al. 2013). ET3 forms a zinc-coordinating dimer Gand delivers the TA protein to the ER where the membrane proteins GET1 and GET2 facilitate insertion. Work in our lab has now led to the identification of the GET pathway orthologues in plants (Figure 2, Xing et al. 2017).
Figure 1: The GET pathway. The pre-targeting complex (PTC) comprising of SGT2, GET4 and GET5 binds the nascent TA protein (green) after emergence from the ribosome followed by transfer of the cargo to GET3. The cytosolic ATPase GET3 shuttles to the ER and delivers the TA protein to the GET1/GET2 receptor complex which facilitates membrane insertion.
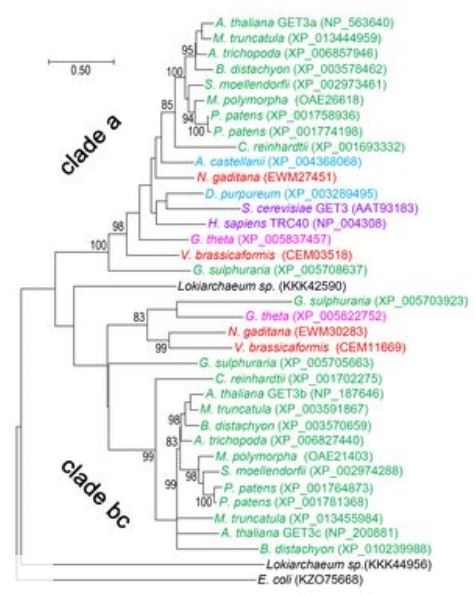
While yeast and mammals only feature a single copy of the cytosolic ATPase GET3, plants and also species of the SAR clade maintain two distinct paralogs (Figure 2, Xing et al. 2017). We found that the recently discovered Lokiarchaeota (Spang et al. 2015) seem to also have two different paralogs which could suggest that Eukaryotes originally kept two copies of GET3 and only lost the second copy in some clades. While the close relative to yeast and mammalian GET3/TRC40 which we termed AtGET3a (At1g01910) localises to the cytosol, AtGET3b (At3g10350) was detected in the chloroplast stroma and AtGET3c (At5g60730) in the mitochondria matrix, respectively. A potential candidate for GET2 remains elusive at present, whereas At4g16444 was identified as putative AtGET1 localising to the ER membrane and interacting with upstream AtGET3a and itself, but not the other AtGET3b/c orthologs (Xing et al. 2017). We also determined a likely candidate for the cytosolic AtGET4 part of the pretargeting complex (At5g63220). While loss of GET function seems not to disturb growth of Arabidopsis plants under laboratory conditions, we uncovered shorter root hairs of T-DNA lines of Atget1, Atget3a and Atget4 – but not Atget3b and Atget3c – when grown on MS media. This phenotype can be rescued through complementation with the respective genes and is not increasing if multiple knockouts are created by crossing. Introducing the marker Qa-SNARE SYP123 (Enami et al. 2009) in Atget1 or Atget3a lines we detected reduced protein levels of GFP-tagged SYP123 at the plasma membrane of root hairs. Loss of SYP123 in Arabidopsis causes reduced root hair growth (Ichikawa et al. 2014) and reduced abundance of this SNARE in the GET pathway knockout lines might be one reason why the root hairs fail to grow normally. Additionally, we also detected reduced SYP123 RNA levels in Atget lines, independent of transgenic or native origin of the transcript.
Overexpression of the cytosolic ATPase AtGET3a - but not AtGET4 - in an Atget1 background leads to germination defects, seedling lethality or severely dwarfed plants, dosage dependent of the amount of AtGET3a present in the cells. While this proves interdependency of AtGET1 and AtGET3, it also highlights - together with the relatively mild phenotype of get lines - the existence of an alternative insertion pathway (for more details please refer to Xing et al. 2017).
Figure 2: Maximum Likelihood, rooted phylogenetic tree of GET3 orthologues revealing two major GET3 branches. 1000 bootstraps were applied, confidence ratios above 70 are included at nodes. Scale bar = changes per residue. Species color code: black, Eubacteria/Proteoarchaeota; purple, Opisthokonta; light blue, Amoebozoa; green, Archaeplastida; red, SAR; magenta, Chromalveolata. (taken from Xing et al. 2017)
The challenges of water scarcity and global food production are interlinked, potentially catastrophically, as the availability of fresh water has a huge impact on future food security. Understanding how plants use and economise this valuable resource is a vital step for future crop improvement. Plants maintain most of their cells in a state of positive turgor pressure; under water deficit, they lose this pressure and wilt. This positive pressure is achieved through an uptake of osmolytes followed by water influx and, during growth, needs to be balanced by an increase in cell surface area and volume. Increasing cell volume requires addition of material and cargo to the expanding cell membrane and wall through vesicle trafficking. An elaborate interplay of protein-protein interactions enables trafficking of the vesicles and their fusion with the plasma membrane. SNARE proteins, which are situated on both vesicle and target membrane, form a tight complex driving the actual fusion event.
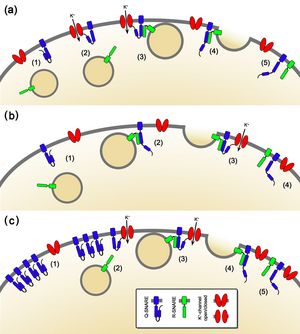
Until recently, SNAREs had generally been associated with their primary role in facilitating membrane fusion. In the past few years we established a novel, functional role for SNAREs in coordinating traffic and transport (Honsbein et al., 2009). The mating-based Split-Ubiquitin System (mbSUS; (Grefen et al., 2007; Grefen et al., 2009; Obrdlik et al., 2004 )), Bimolecular Fluorescence Complementation (BiFC ( Hu et al., 2002 )) and co-immunoprecipitation analysis demonstrated a physical interaction of the Qa-SNARE SYP121 with the K+-channel subunit KC1. Closely related homologues of SYP121 failed to interact. However, other Shaker channels such as KAT1 are also able to bind SYP121 (Honsbein et al., 2011). Interestingly, neither expression levels, nor subcellular localisation of K+-channels, i.e. insertion in the plasma membrane, are altered in the absence of SYP121. Because channel traffic per se appears normal in these circumstances, the effects on channel activity are most easily explained through non-canonical regulation via the SNARE. Voltage-clamp experiments in Xenopus oocytes revealed that coexpression with SYP121 altered the gating behaviour of the AKT1-KC1 heterotetramer. This complex forms the main channel pathway in roots for K+-uptake. Under K+-limiting conditions the syp121 line shows reduced growth phenocopying the akt1, and kc1-knockout mutants. This is further corroborated through electrophysiological recordings of impaled roots, revealing the loss of inward current in the syp121, akt1 and kc1 knockout lines (Honsbein et al., 2009).
Figure 3: Binding of SYP121 with a K+ channel may (a) drive conversion to the so-called ‘open’ conformation of the Qa-SNARE, thereby promoting SNARE complex formation, or (b) like SM proteins stabilise the SNARE complex once formed to facilitate fusion. (c) Transient association of SYP121 with the K+ channels could ensure a dynamic pool of SNAREs primed and available for vesicle fusion without tying up the much smaller pool of channels present at the membrane. (Figure taken from Grefen et al. 2011)

Given the established role of SNARE proteins in membrane fusion events, our findings in plants were rather unexpected. We therefore speculated that the SNARE channel interaction might act like a molecular governor helping to fine-tune the interplay of membrane-trafficking and ion transport (Grefen and Blatt 2008). Looking deeper into the mechanism of the interaction using chimeric proteins and Alanine-scanning site-directed mutagenesis (Karnik et al., 2013) we identified the interacting domain of both channel (see below) and SNARE protein ( Grefen et al., 2010). Exchanging domains between the interacting SYP121 and its closest, non-interacting homologue SYP122 allowed narrowing down the site of binding to the first 40 amino acids close to the N-terminus of the SNARE. Further single-site mutations helped to identify a stretch of 4 amino acids, the “FxRF-motif”, which is essential for binding and in vivo functionality of the interaction (Grefen et al., 2010). Interestingly, the FxRF-motif is highly conserved in dicotyledonous plants amongst orthologues of SYP121. A preliminary scan of Monocots and other plant species showed only weak conservation of the motif therein (Figure 4).
Figure 4: The FxRF motif is only conserved in DicotsGiven the established role of SNARE proteins in membrane fusion events, our findings in plants were rather unexpected. We therefore speculated that the SNARE channel interaction might act like a molecular governor
| The canonical role of SNAREs in facilitating membrane fusion events has been widely established and accepted. However, our findings have changed the view of SNARE proteins as being solely involved in SNARE-complex formation. The interaction with K+-channels has opened a new dimension to SNARE function and we are only just beginning to understand how traffic and transport might be regulated coordinately. Research in our group focusses on elucidating the mechanisms of SNARE-channel interactions and its consequences for plant growth and development (see also Grefen et al. 2015, Zhang et al 2015). |

A lot of our previous and future research is based on protein-protein interaction studies (Xing et al., 2016). We have developed and optimised techniques such as expanding the Split-Ubiquitin System (SUS; (Obrdlik et al., 2004; Grefen et al., 2007; Grefen et al., 2009)) to be able to detect multimeric interactions.
Also, we have devised a novel, Gateway-compatible cloning system named 2in1 that enables single-step transfer of two independent genes into two different expression cassettes on the same vector backbone and demonstrated how this concept ensures the credibility of in vivo protein-protein interaction methods by its application in our ratiometric bimolecular fluorescence complementation assay (rBiFC; (Grefen and Blatt, 2012b)). The simultaneous introduction of both fusion proteins on the same plasmid ensures equal gene dosage, and the inclusion of an internal fluorescence marker allows for expression control and ratiometric quantification.
Biotechniques front cover of November 2012 featuring our rBiFC method.
While the expansion of the SUS, the SUS bridge assay (SUB; (Grefen 2014; Grefen and Blatt, 2012a)) will help to resolve multimeric interaction partners the ratiometric approach in BiFC will enable dissecting different interaction strength.