Vesicle trafficking is facilitated by a complex interplay of protein-protein interactions. Key players are the so-called SNARE proteins (Soluble N-ethylmaleimide-Sensitive Fusion Protein Attachment Protein Receptor) that enable the actual fusion through a tight interaction that helps to overcome the strong dehydration forces associated with lipid bilayers in an aqueous environment (Jahn et al., 2006).
A distinctive feature is the SNARE domain, an α-helical heptad repeat forming a coiled-coil structure with a conserved Glutamine (Q) or Arginine (R) residue at its “zero” position subdividing this protein family into two subclasses: Q- and R-SNAREs. Another terminology uses the terms t-SNARE (target-membrane) and v-SNARE (vesicle) according to their subcellular localization. However, this terminology might prove confusing when homotypic fusion events are analysed. The SNARE core complex that drives membrane fusion consists of four SNARE motifs, three Q and one R-SNARE motif. Q-SNAREs are further subdivided into Qa-, Qb and Qc-SNAREs one of each being essential to form the SNARE core complex that enables membrane fusion (see figure 5).
Interestingly, plant genomes encode twice as many SNAREs than humans; however, the majority of the plant SNAREs are not novel per se but rather arise from gene duplication events that have led to a greater number of homologues suggesting the need for more diverse or specialized roles (Lipka et al., 2007).
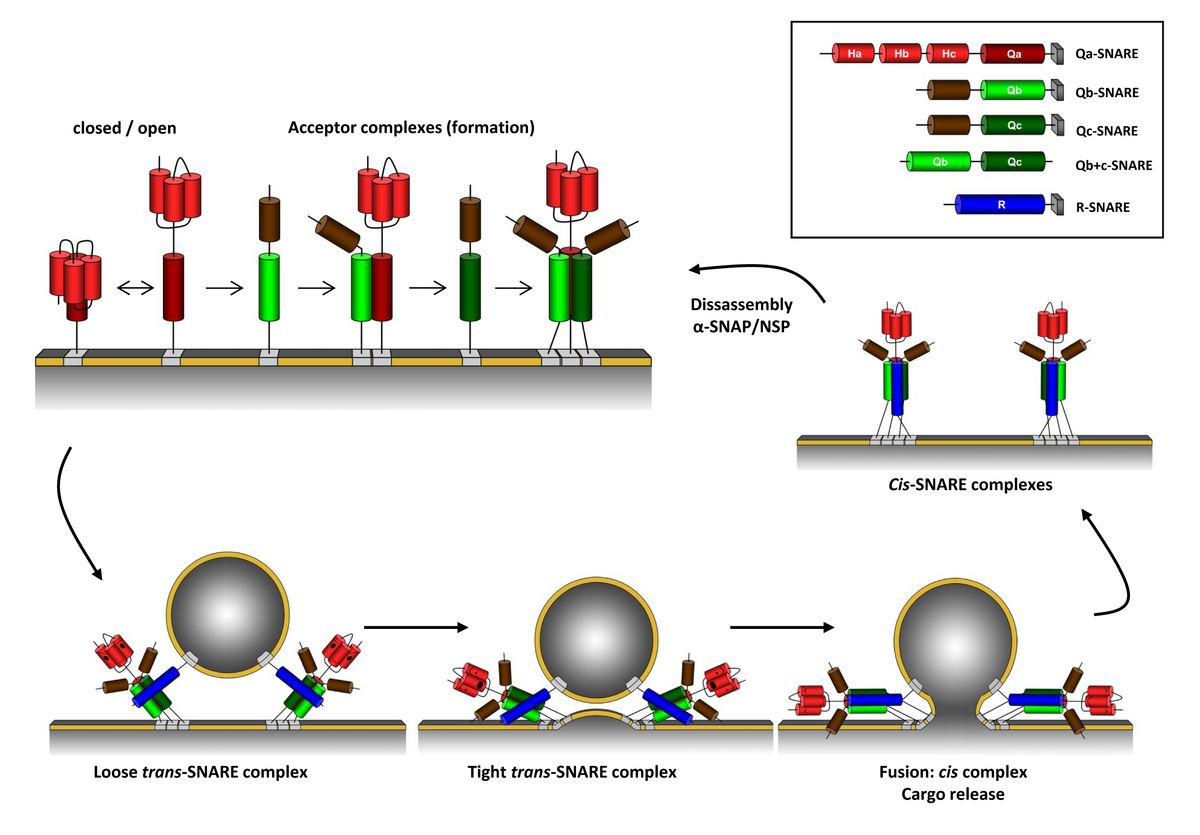
Figure 5: Elements of the SNARE complex differ widely in size and structure, but share common structural motifs that contribute to interactions at the complex core. Within the bundled α-helices of the SNARE core complex, at least one of each is derived from a membrane-anchored protein associated with the target membrane (t-SNARE) and with the vesicle (v-SNARE). Functional SNARE complexes are built of one element of each of four submotif domains, designated Qa, Qb, Qc and R, defined by a glutamine or arginine residue at the centre of the submotif. The amphipathicity of these submotifs strongly favours their assembly like a semi-crystalline ‘zipper’ (Jahn et al. 2003). Here the SNARE components are colour-coded blue (RSNARE), red (syntaxin-like Qa-SNARE), light green (Qb-SNARE), and dark green (Qc-SNARE). Structural details are simplified for visual clarity. The cycle begins with release of the closed conformation with the Ha/Hb/Hc domain (light red) to expose the Qa-SNARE (H3) motif (dark red) and form an acceptor complex. Association in the trans-complex with the R-SNARE is accompanied by a large increase in core a-helical structure that drives transition to the cis-complex. Dissociation of the cis-complex and re-priming requires the energy input of ATP hydrolysis and is achieved through the binding of a-SNAP and the NSF ATPase. Although some SNARE complexes are an assembly of three proteins – for example, in neurons comprising VAMP (R) and Syntaxin1A (Qa), with SNAP25 contributing two submotifs (Qb and Qc) – it is more common for each SNARE partner to contribute a single submotif. (Figure modified from Grefen and Blatt 2008)
Potassium is an essential macronutrient for plants (White et al., 2010); it constitutes the major inorganic, osmotic solute that drives cell expansion. It also regulates physiological responses towards environmental stresses. One of the most important functions and most studied is the opening and closing of the stomata which is achieved through turgor changes that follow K+-uptake and release. K+-transport through plasma membranes can either be facilitated by channels or secondary transporters.
Plants have evolved 3 different types of K+-channels, Kv-like, Tandem-Pore K+-channels (TPK) and the K+-inward rectifier (Kir) channels (Lebaudy et al., 2007). All three have orthologues in animals. The first channels identified through yeast complementation were members of the Kv-like family. The Arabidopsis genome encodes 9 Kv-like channels, of which 4 are inward rectifying (KAT1, KAT2, AKT1, SPIK), 2 outward-rectifying (GORK, SKOR), 1 silent (KC1) and 2 which are weak or unknown rectifying channels (AKT2/3, AKT5) (Pilot et al., 2003).
Each channel consists of 6 transmembrane α-helices (S1-S6) and incorporates a pore (P) loop between the S5 and S6 helices (see figure 6A). A functional channel is assembled by four α-subunits. These tetramers can consist of homomeric as well as heteromeric complexes adding another layer of regulatory potential to K+-transport (Dreyer et al., 1997; Lebaudy et al., 2007). The channels are voltage-gated; thus, they are regulated through changes in plasma membrane polarisation. The first four α-helices form the voltage sensor, the S4-domain containing regularly spaced, positively charged amino acids (arginine and lysine). The gate is formed as diaphragm between the base of the four S5-S6 helices, and voltage leads to a positional change of the voltage sensors which then draw open this diaphragm (see figure 6B).
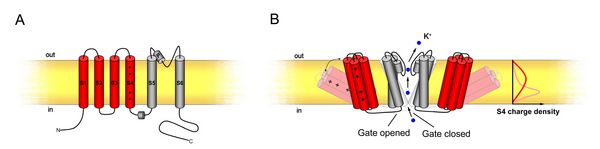
Figure 6: Shaker-like potassium channels. (A) Domain structure of an α-subunit. (B) Opening of the channel allowing K+-ions to pass, showing the leaver-like mechanism of the voltage sensor. (Figure taken from Grefen and Blatt 2008), details in text.
One of the best known in vivo protein-protein interaction systems is the Yeast-2-Hybrid (Y2H) system which was introduced by Fields and Song in 1989. It is based on the artificial cleavage of the yeast’s GAL4 transcriptional activator into two functional domains: the N-terminal DNA binding domain (BD) and the C-terminal activation domain (AD). Fusion of proteins of interest with either of those two domains and coexpression in specialised yeast cells (GAL4 knockout, integrated reporter constructs, etc.) allows for the detection of interaction. Reconstitution of the GAL4 halves through close proximity of the two proteins of interest enables reporter gene activity to be monitored. The Y2H system has become a tremendously successful method and a huge variation of its original design has since been established. An abundance of reviews has been published that also details the issues of the method. One major flaw is the necessity of the fusion proteins to be localised to the nucleus as the reconstituted GAL4 needs to bind to the DNA upstream of the reporter genes. This requires for example membrane proteins to be truncated before interaction assays can be performed. It also means that interaction takes place dislodged from its native environment. In 1994, the split-ubiquitin system, an alternative method that overcomes this flaw was developed by Johnsson and Varshavsky; that was only 5 years after the Y2H had been established, yet it never gained as much attention (a google search using “yeast two hybrid” yields 1.5 million hits, “Split Ubiquitin” around 25.000, see also Xing et al. 2016).
Despite this disregard of the scientific community, the split-ubiquitin system (SUS, see figure 7) in yeast is a very reliable tool to detect and dissect protein-protein interactions of full length proteins. It is based on splitting the yeast’s ubiquitin protein into an N- (Nub) and C-terminal (Cub) half, the latter being additionally tagged with a hybrid transcription factor (PLV = ProteinA-LexAVP16) and fused to proteins of interest (Johnsson et al., 1994; Stagljar et al., 1998). To prevent spontaneous reassembly of Nub and Cub, the N-terminal half is mutated at a key residue (Ile13 to Gly = “NubG”). Upon interaction of the fusion proteins Nub and Cub reassemble, are recognised by ubiquitin specific proteases and the transcription factor is released to activate reporter genes that confer growth on depleted media or β-galactosidase activity. We have extensively worked with the SUS in the past and benefit from a range of different vectors with modified tag orientations, different promoters and origins. Feel free to contact us if you would like to work with the SUS or if you simply need some troubleshooting. Browse our Resources page for vector maps, sequences and protocols.
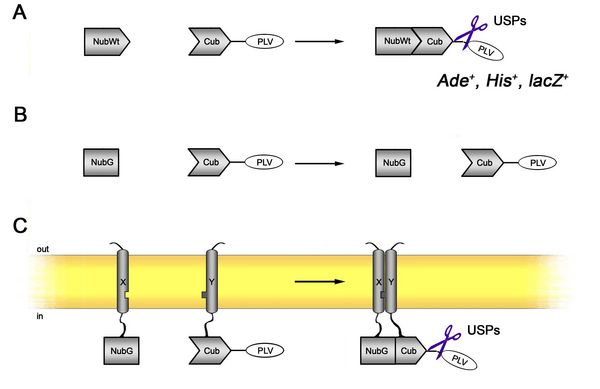
Figure 7: The split-ubiquitin system. (A) The N- and C-terminal halves of the yeasts’ ubiquitin are used for this protein-protein interaction tool. Reassembly of the two halves allows for recognition by ubiquitin specific proteases (USPs) and cleavage of a hybrid transcription factor (PLV) which activates reporter genes. (B) Mutation of NubWt to NubG prevents spontaneous reassembly. (C) Fusion of two (here:) membrane-bound interacting proteins forces Nub and Cub together and subsequently leading to reporter gene activity.
We have recently established a novel technique, the SUS bridge assay (SUB) that allows analysing multimeric interactions (Grefen 2014; Grefen and Blatt 2012a) (see figure 8). The SUB assay is a powerful expansion of the classical SUS by simply expressing an additional protein along with the bait and prey pair. Three different types of interactions can hence be detected:
A) an interaction between a bait and prey pair that do not interact could be facilitated through coexpression of a third, bridging protein;
B) an interaction between a bait and prey pair that do interact could be inhibited through a third protein or
C) an interaction could be enhanced.
The proof-of-concept of the method has recently been published (Grefen 2014; Grefen and Blatt, 2012a; Honsbein et al., 2009), and we have established a range of Gateway™-compatible expression vectors to test inducible or repressible expression of third proteins.

Figure 8: The SUS bridge assay (SUB). Expression of a third untagged protein (here: Z) enables the detection of a trimeric interaction. It facilitates (A) an interaction between two proteins that do not interact, inhibits (B) or enhances (C) an existing interaction. (Figure taken from Grefen 2014)
Analysing higher order protein complexes requires introduction of multiple plasmids into a host organism. This becomes cumbersome and to a certain extent unreliable as it cannot be guaranteed that all plasmids will be transformed into a single cell. We have devised a novel, Gateway-compatible cloning system - 2in1 - that enables single-step transfer of two independent genes into two different expression cassettes on the same vector backbone (Grefen and Blatt 2012b).
This 2in1 system can be readily transferred in other cell and expression systems (Hecker et al. 2015). We have created a set of helper vectors similar to Life Technologies’ Gateway conversion kit that allows conversion of any expression vector providing high flexibility for the researcher.
The use of the 2in1 cloning system is not limited to improving protein-protein interaction methods; this concept offers advantages for all techniques that depend on multiple gene expression, by generally circumventing issues inherent to work with individually cloned genes (Karnik et al. 2013b). It allows increasing the number of genes to be introduced in a foreign organism as it decreases the number of plasmids, and thereby auxotrophies and other selection markers, necessary for the purpose
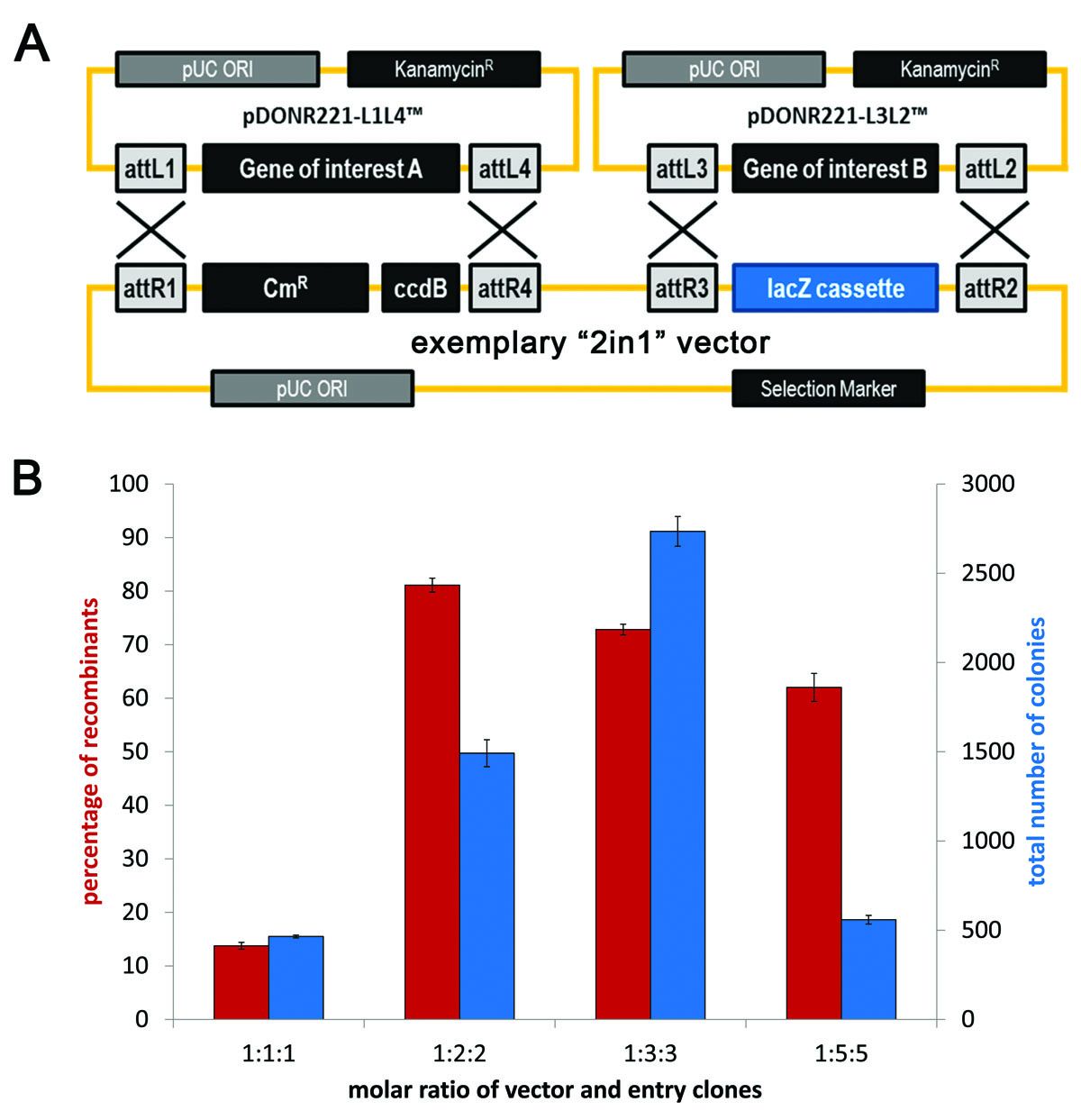
Figure 9: (A) Vector maps illustrating the 2in1 concept with its recombination reactions between two entry vectors and an exemplary 2in1 destination vector carrying the two independent cloning cassettes. (B) Recombination efficiency and total colony numbers obtained with different molar ratios of vector and entry clones. (Figure composite from Grefen and Blatt 2012b)
BiFC is a versatile technique for detection of protein-protein interactions in vivo ( Hu et al., 2002 ). A fluorophore such as YFP is split into two non-fluorescing halves which are used to tag proteins of interest. Interaction of those proteins is monitored by assaying the fluorescence intensity. Absence of fluorescence is usually interpreted as a lack of interaction. BiFC however, has a couple of significant shortcomings that have not been addressed so far in a systematic way. Conventional BiFC lacks an internal reference marker; thus it is at best cumbersome to distinguish weak interactions from background fluorescence levels. Also, using two independent fusion proteins does not account for one being weaker expressed, resulting in misinterpretation of results, i.e. absence of or a seemingly weaker interaction does not necessarily reflect the true nature of that particular protein couple. Even Western data can not sufficiently exclude the possibility of differential expression in individual cells which are monitored for interaction ( Xing et al., 2016 ).
Through merging the BiFC with the novel “2in1” cloning system some previous issues of the technique will be avoided ( Grefen and Blatt, 2012b ). Being able to express both fusion proteins from the same T-DNA will allow expressing similar ratios (see figure 10, Hecker et al. 2015 ). In addition, a third expression cassette containing a fluorescent cytosolic marker (here: RFP) will act as an individual transformation control identifying cells that express the transgenes. This is particularly useful if a protein pair does not interact as each fusion construct alone is non-fluorescent. However, the major advantage of constitutive RFP expression is the possibility to quantify BiFC results. Through measuring mean fluorescent intensities of both fluorophores, a ratio can be calculated that will allow putting results from different protein pairs into context, grading different interaction strengths in a semi-quantitative approach. Additionally, myc and HA epitopes at the YFP-halves allow verification via immunoblot.
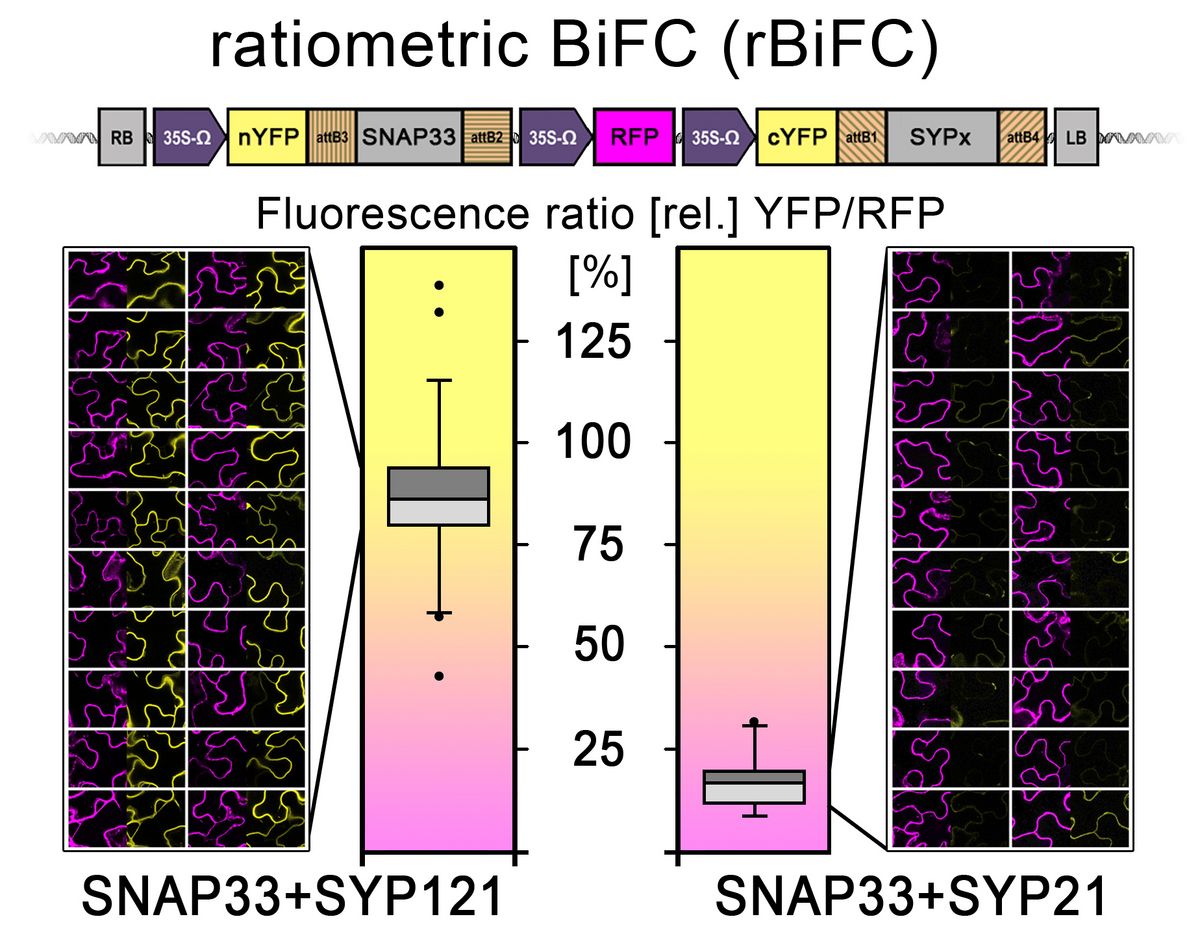
Figure 11: Exemplary rBiFC experiment with T‐DNA cartoon at the top (data from Hecker et al., 2015). Cytosolic nYFP‐SNAP33 and either plasma membrane localized cYFP‐SYP121 or PVC/tonoplast membrane localized cYFP‐SYP21 were coexpressed from a single contiguous T‐DNA including a reference RFP marker (Grefen and Blatt, 2012). Ratio between complemented YFP to RFP was calculated from 20 entire individual images each recorded at the interfaces of two to three cells to avoid bias arising from picking single cells. As the interaction is at the cell periphery, imaging of the nucleus was prevented to avoid a disproportionate increase in detection of soluble RFP which migrates into the nucleus and would bias this particular assay. Data from 20 independent YFP/RFP ratios shown as box plot diagrams (the light grey box represents the 25‐50% range, the dark grey box the 50‐75% range of where all values lay). Whiskers mark the 1.5x interquartile range and black dots outliers. (Image taken from Xing et al., 2016)
Site-directed Mutagenesis (SDM) is an indispensable tool for structure-function studies. Through a simple PCR reaction with overlapping, complementary oligonucleotide pairs virtually any desired mutation can be introduced in a plasmid-borne gene. The reaction mixture is used to transform E. coli and colonies are screened for mutants through sequencing. To avoid recovery of unmutated template DNA, the PCR reaction is incubated with the restriction endonuclease DpnI which specifically targets and cleaves methylated DNA (see Figure 12).
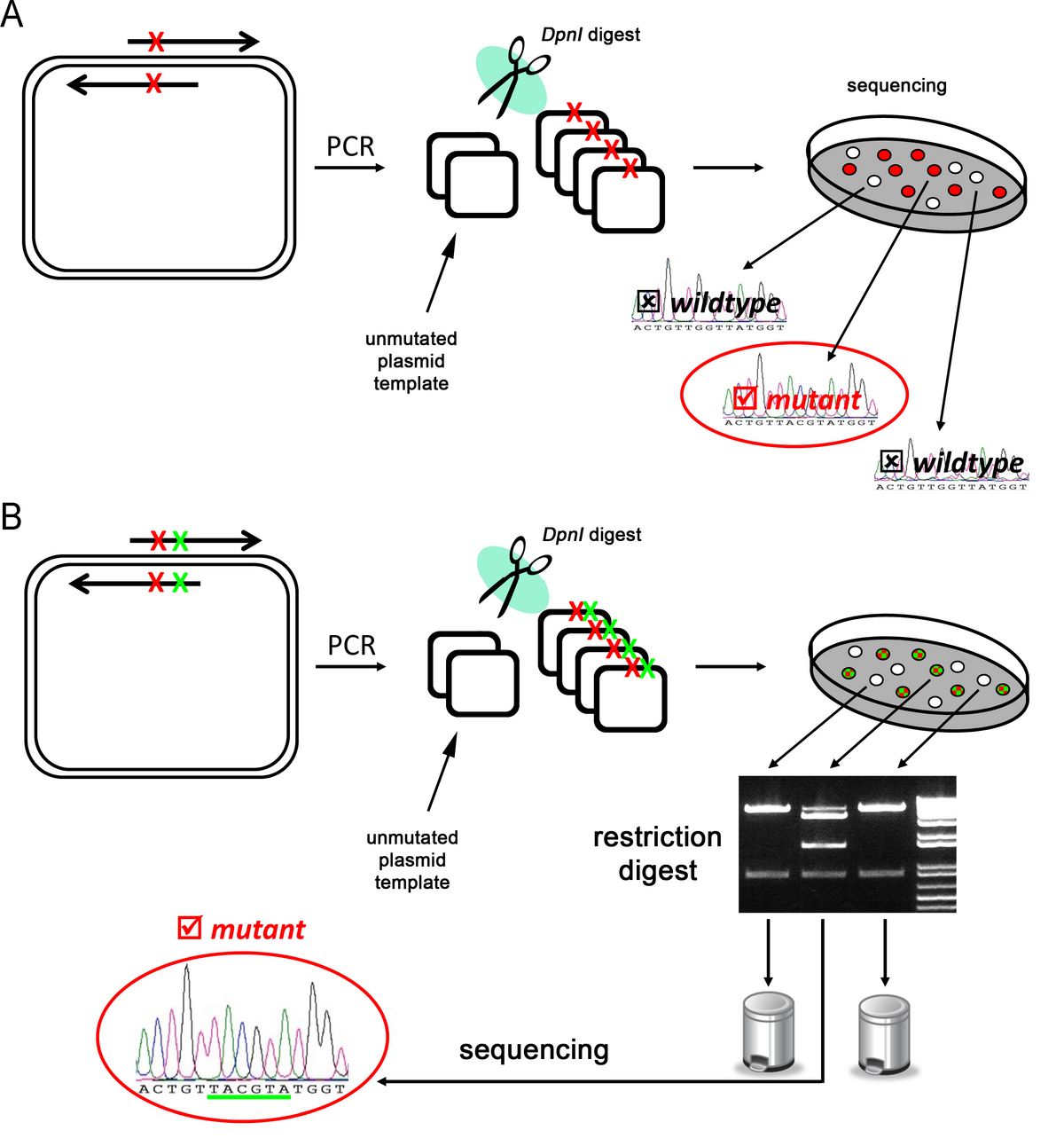
Figure 12: SDM workflow (A) SDM using a primer pair that includes the desired mutation (red “x”). Identification of positively mutated clones can only be done via sequencing. (B) Introducing an additional silent mutation (green “x”) allows pre-screening of colonies via restriction analysis. (Please note: The colour of the colonies is for visualising purposes only. In reality, all colonies look the same, hence it is not possible to distinguish mutated and template plasmid at this stage)
Since PCR does not introduce methylation of the DNA this method reduces the amount of template DNA. However digest with DpnI is sometimes incomplete and does not include cleavage of hemimethylated DNA (template plus mutated strand). An evaluation of all mutated clones generated through SDM in the past five years in our lab (more than 100 different constructs) revealed that a rescue of 20-30% of un-mutated, wildtype plasmids after DpnI digest is inevitable. This means screening more colonies via sequencing which is time-consuming and costly. For that reason some researchers have decided to introduce silent mutations in addition to the desired point mutation which do not alter the protein but allow distinction on DNA level through restriction sites. Designing an oligonucleotide which fulfills this requirement is hard work with pencil and paper and requires skill and time. The silent mutation needs to take into account the frame as well as finding a potential palindromic sequence that is recognized by a certain restriction endonuclease.
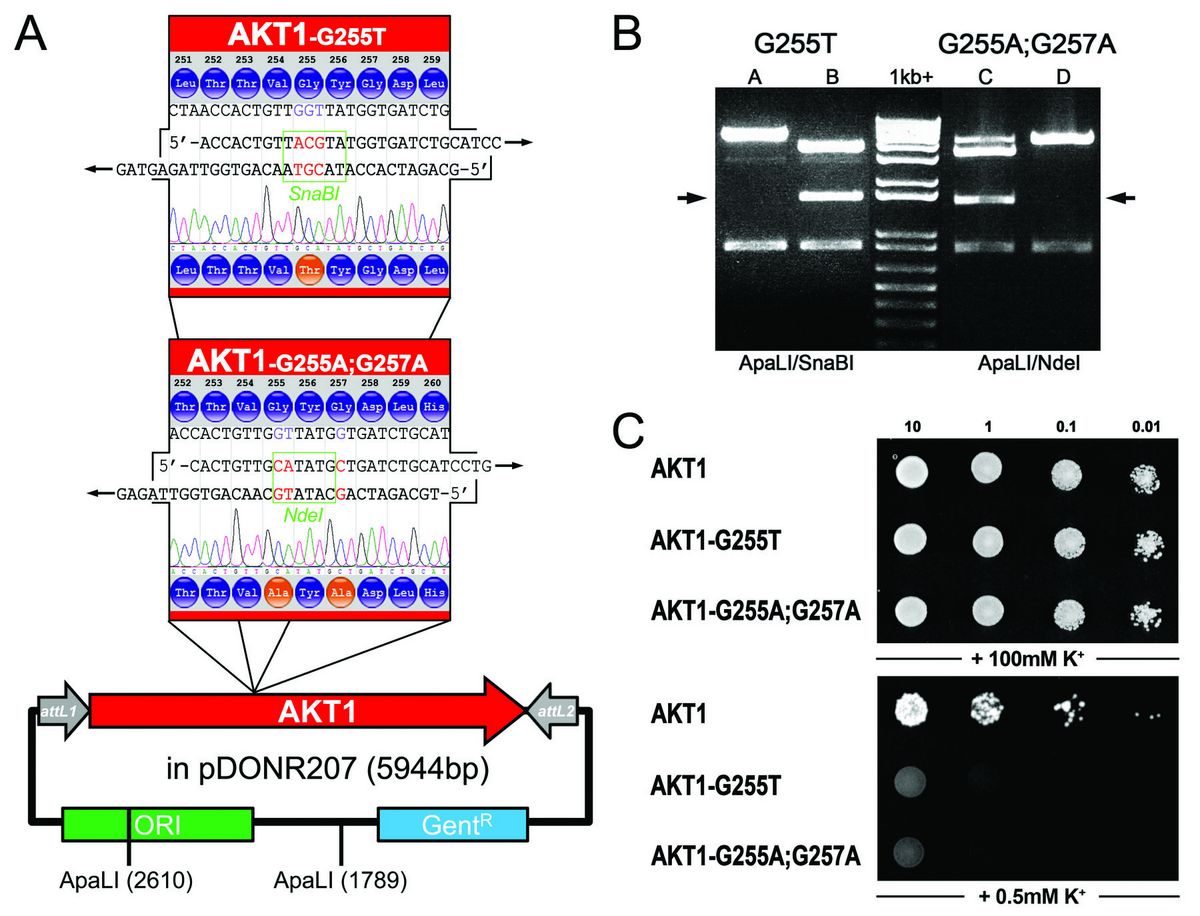
Figure 13: Mutating the pore domain of the Arabidopsis potassium channel AKT1 and functional consequences in vivo. (A) Wild type AKT1 in the Gateway® Entry vector pDONR207 is used as template for SDM of the GYG-motif within the pore-domain of the voltage-gated potassium channel AKT1 which is critical for formation of the channel-pore. SDM-Assist suggested primers for a single mutation of the Glycine 255 to Threonine adding a SnaBI-site, and for mutating both Glycines to Alanine introducing an NdeI site within the gene. Both restriction sites were absent in the wild type sequence. (B) DNA restriction analysis of randomly picked colonies after SDM demonstrates that distinction between wild type (lane 1 and 4) and mutated sequences (lane 2 and 3) becomes easily possible through the addition of a restriction site: an additional band appears in mutated plasmids (arrows). Sequence verification (trace file) is incorporated in (A). (C) Functional consequence of the single or double mutation: The yeast strain SGY1528 (Tang et al. 1995) which is disrupted in potassium uptake can only grow on high K+-medium (+100mM) or on low K+-medium (0.5mM) if an exogenous K+ transporter (such as wild type AKT1) is expressed. Disruption of the GYG-motif in the mutant AKT1-G255T and G255A;G257A renders the channel dysfunctional on low K+-medium, the yeast fails to grow. Equal amounts of yeast were dropped as dilution series on CSM-Leu-, His-, Trp- media with final potassium concentrations of 100mM or 0.5mM. Growth on plates with high K+-concentration was monitored after 2 days and on low K+-plates after 8 days at 30°C. AKT1 and mutants were expressed using the yeast vector pMetYC-Dest. (Figure taken from Karnik et al. 2013)
We have programmed a software that allows for simple, reliable and fast detection of SDM primers. SDM Assist will guide the user in three easy steps through the process of primer design allowing to drag and drop the desired mutation directly on the protein sequence, enabling to pick from different “silent” restriction sites and finally offering a range of primers ranked by their thermodynamic parameters. The software is a stand-alone freeware and can be downloaded here. There is also an online video tutorial that provides guidance. For more information download Karnik et al. 2013a or contact us directly.
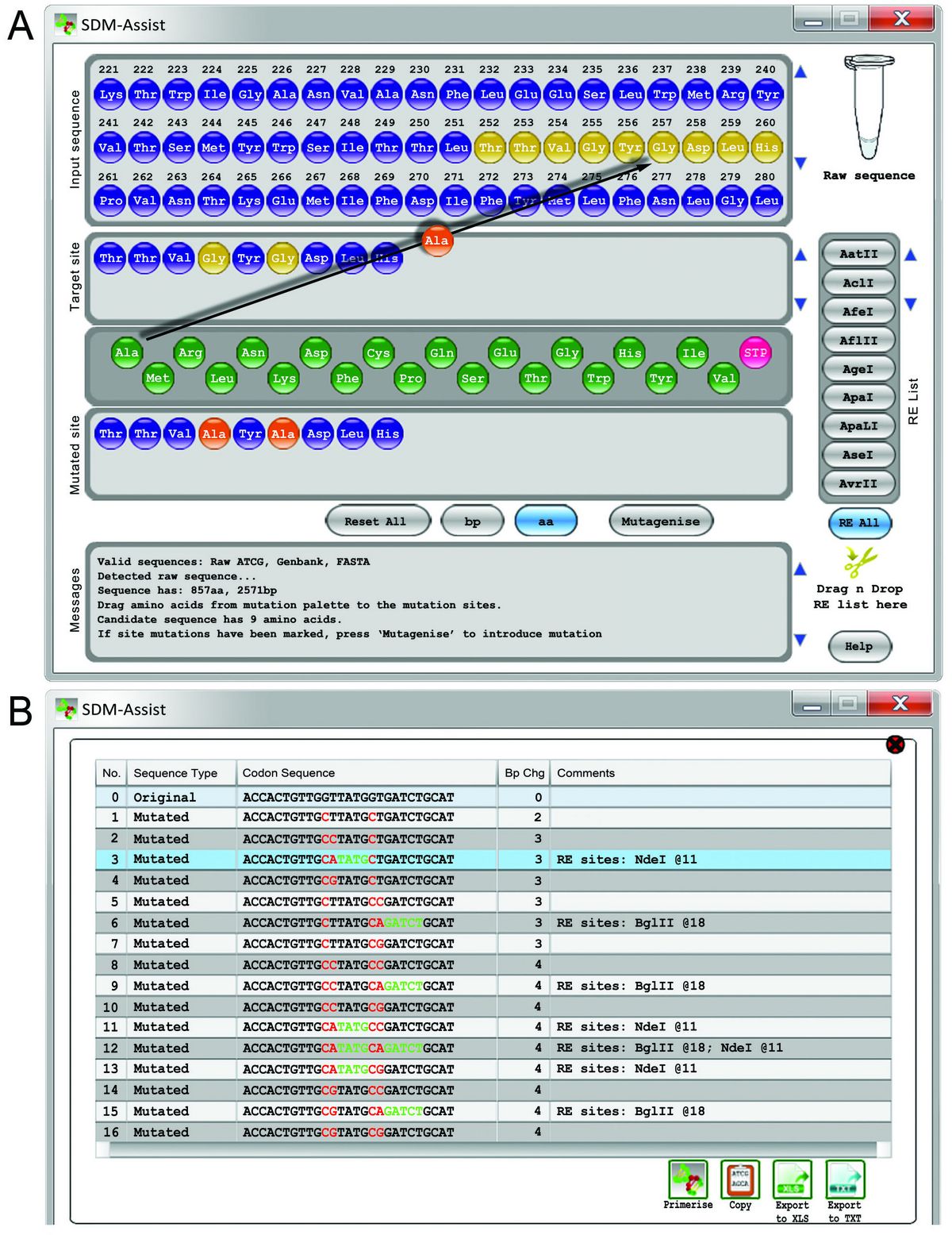
11: Screenshots of SDM-Assist while creating primers to mutate the Arabidopsis potassium channel AKT1. (A) Dragging and dropping of Alanine onto the Glycine residues at position 255 and 257 (black arrow) of the AKT1 sequence creates a stretch of 9 amino acids (below, “Mutated site”) that includes Alanine (orange) instead of Glycine (yellow) residues. Pressing the “Mutagenise” button opens a new window (B) that includes all possible nucleotide sequences derived from those 9 amino acids. The table highlights the mutated nucleotides in red and resulting palindromes which create a restriction site in green. The user has to choose one of the mutated sequences and press the button “primerise” to get a list of potential primers. (Figure taken from Karnik et al. 2013a)